Abstract
Background: Malglycemia, defined as hypoglycemia, hyperglycemia, or glycemic variability is common in adult hematopoietic stem cell transplant (HSCT) recipients. It is associated with increased rates of infection, acute graft-versus-host disease (GVHD), organ dysfunction, time to engraftment, and mortality. Malglycemia in pediatric HSCT has not been well studied and may have unique incidence, risk factors and clinical implications. Therefore, this study aimed to identify the incidence of and risk factors for malglycemia inpediatric, adolescent and young adult (AYA) HSCT patients, and to characterize associated impacts on outcomes.
Methods: Medical records for 351 pediatric/AYA patients, age 0-26 years (average 8.0 years, interquartiles 3.0, 15.0; 59.3% male), who underwent first HSCT from 2007-2016 at Children's Hospital Colorado were retrospectively reviewed. Patients with pre-existing diabetes mellitus (n=5) and patients with inadequate blood glucose data (n=2) were excluded, resulting in a final cohort of 344 patients. The goal was to identify risk factors for malglycemia and to determine its association with clinically significant infection in the first 100 days after HSCT. Malglycemia was defined as presence of mean blood glucose ≥126 mg/dL and/or standard deviation ≥29 mg/dL for each patient, assessed for the following intervals: pre-HSCT (days -14 to 0), days 0-30, days 0-100, and overall (days -14 to +100).
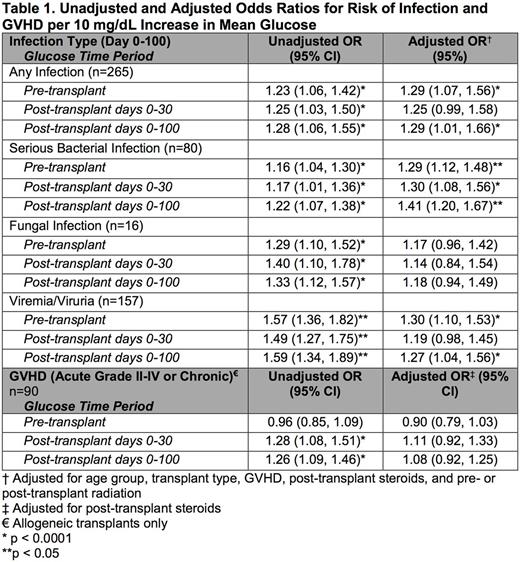
Results: Malglycemia occurred in 43.9% of patients, with 22.3% experiencing malglycemia in the two weeks prior to HSCT, 22.3% in the first 30 days post-HSCT, and 22.6% in the first 100 days post-HSCT. In univariate analysis, the rate of malglycemia was higher among patients who underwent allogeneic than autologous transplant (pre-HSCT 35.6% vs 8.4%, p<0.001; days 0-30 25.8% vs 16.1%, p=0.056; days 0-100 27.6% vs 13.6%, p=0.004). In multivariate analysis, age ≥12 years was a risk factor for pre-HSCT malglycemia (p<0.0001), and post-HSCT steroids and treatment with tacrolimus were risk factors for post-HSCT (day 0-100) malglycemia (p<0.0001, p=0.034). Additional factors that did not have an association with malglycemia in multivariate analysis included body mass index, underlying diagnosis category (malignant vs non-malignant), pre-HSCT treatment with insulin, asparaginase, steroids, or radiation, HSCT type (autologous vs allogeneic), preparative regimen, post-HSCT treatment with total parenteral nutrition or radiation. Malglycemia at any time was associated with increased infection (p=0.008-0.042). After adjusting for age, pre-HSCT radiation, HSCT type, GVHD diagnosis (grades II-IV and/or chronic) and post-HSCT steroids, for each 10 mg/dL increase in pre-HSCT glucose, there was a 29.3% increase in any post-HSCT infection (p=0.007) and specifically a 28.8% increase in serious bacterial infection (SBI) (p<0.0001) and a 29.7% increase in viremia/viruria (p=0.002); similar findings existed at other time intervals analyzed (Table 1). Additionally, after adjusting for HSCT type and presence of GVHD, for every 10 mg/dL increase in mean glucose over days 0-100, the average number of hospitalized days from 0-100 increased by 2.7 days (p<0.0001) and days in the intensive care unit (ICU) increased by 1 day (p<0.0001). Similarly, patients with malglycemia during days 0-100 averaged 12.9 more hospital days (p<0.0001) and 3.9 more ICU days (p<0.0001) in the first 100 days post-HSCT than patients without malglycemia, regardless of HSCT type or GVHD diagnosis.
Conclusion : Malglycemia occurs frequently in the pediatric/AYA HSCT population and is more common in adolescent patients. When controlling for HSCT type, presence of GVHD and treatment with steroids, malglycemia is an independent risk factor leading to increased rates of serious bacterial infections and viremia/viruria. Mechanistically, the temporal relationship between pre-HSCT mean glucose increases and higher rates of infection support a causal link. The underlying pathophysiology by which malglycemia may influence outcomes such as infection is likely multifactorial, but data suggests that hyperglycemia drives immune system impairment and supports a pro-inflammatory state. Understanding the morbidities associated with malglycemia, and the underlying pathophysiology, should inform future studies evaluating the utility of intensive glucose control in improving pediatric/AYA HSCT outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal